APPROVAL
I. Release Activity
a. Research and development purposes in all field experiments
b. Supply or offer to supply for sale or placing on the market
c. Offer as gift, prize or free item
d. Disposal
e. Remediation purposes
f. Any other activity which does not amount to contained use
II. Application
a. Apply to release activities and import activities involving living modified organisms.
b. Not apply to the importation of living modified organisms intended for purposes of undertaking a contained use activity.
III. Application Approval
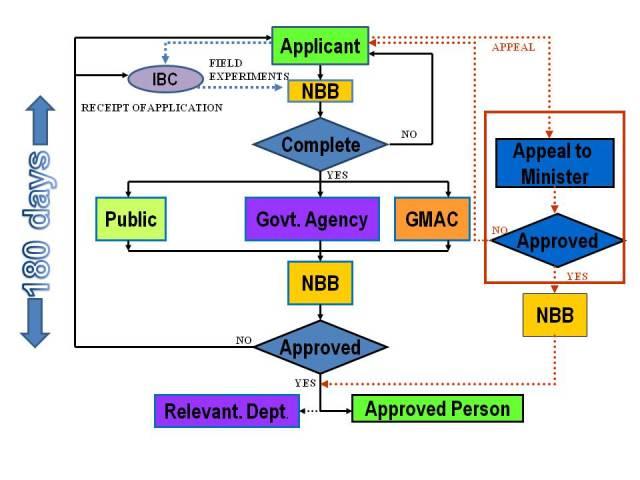
a. An application for the approval of any release activity, or any importation of living modified organisms, or both shall be submitted to the Director General through the institutional biosafety committee in the prescribed manner, together with the prescribed fees, and shall be accompanied with:
• risk assessment and a risk management report
• emergency responses plan
• other information as may be specified by the National Biosafety Board (NBB)
b. Upon receiving the application the DG shall
• Refer it to Genetic Modification Advisory Committee (GMAC) for its recommendations,
• Refer it to relevant government agencies for specific matters
• Invite public participation for purpose of public disclosure
c. GMAC shall forward its recommendation whether or not the application should be approved and the terms and conditions to be imposed by the NBB, if any, after the assessment.
d. After having considered the recommendations of the GMAC, the comments of the relevant department or agency, the views of members of the public, if any, and any additional information, the NBB may grant the application by issuing a certificate of approval or refuse the application.
IV. Certificate Approval
a. The Board may grant the application by issuing a certificate of approval or refuse the application.
b. The Board may impose such terms and conditions as the Board thinks fit.
IV. Validity
a. Approval shall be valid for the subsequent similar release activity involving the same living modified organisms or products of such organisms or importation involving the same living modified organisms undertaken by such approved person.
b. "Same living modified organisms or products of such organisms" means the living modified organisms or products of such organisms as described in the approval and involving the same transformation event.
VI. Approval Process
VII. Approval Fees
For release activities, the Regulations prescribe the fees as follows:
i. For R&D purposes in all field experiments per release site:
- less than 5 hectares – RM100
- 5 hectares to 10 hectares – RM250
- More than 10 hectares – RM500
ii. All release activities other than above – RM5000.
The fees must be paid by money order or bank draft in the name of the Secretary General of the Ministry of Natural Resources and Environment.
Please email This email address is being protected from spambots. You need JavaScript enabled to view it. for more information
VIII. Approval Forms
- Form A APPROVAL FOR RELEASE ACTIVITIES OF LMO (RESEARCH AND DEVELOPMENT PURPOSES IN ALL FIELD EXPERIMENTS) OR IMPORTATION OF LMO THAT IS HIGHER PLANT
- Form B APPROVAL FOR RELEASE ACTIVITIES OF LMO (SCRESEARCH AND DEVELOPMENT PURPOSES IN ALL FIELD EXPERIMENTS) OR IMPORTATION OF LMO OTHER THAN HIGHER PLANTS
- Form C APPROVAL FOR RELEASE ACTIVITIES (SECOND SCHEDULE 2-6) OR IMPORTATION OF LMO THAT IS A HIGHER PLANT AND PRODUCT OF SUCH ORGANISM
- Form D APPROVAL FOR RELEASE ACTIVITIES (SECOND SCHEDULE 2-6) OF LMO OTHER THAN A HIGHER PLANT AND PRODUCT OF SUCH ORGANISM
• Risk Assessment Matrix: Click Here
• Standard Operating Procedures: Click Here
Please sent your application to This email address is being protected from spambots. You need JavaScript enabled to view it.
NOTIFICATION
I. CONTAINED USE
Any operation including R&D, production or manufacturing operation involving LMOs, or storage of LMOs, undertaken within a facility, installation or other physical structure such as it prevents contact and impact of the LMOs on the external environment.
II. APPLICATION
a. Apply to exportation of living modified organisms
b. Contained use involving living modified organisms
c. Importation of living modified organisms for purposes of undertaking a contained use activity
III. SUBMISSION OF NOTIFICATION
The notification shall be submitted to the Director General through the Institutional Biosafety Committee in the prescribed form and accompanied by the following documents:
a. An emergency response plan
b. Specific measures for the contained use activity (regulation + IBC)
c. Such other information as may be specified by the Board
d. Compliant with requirements of importing country (export)
IV. ACKNOWLEDGEMENTS
a. The Director General will issue an acknowledgement of receipt and the person to whom such acknowledgement is issued may undertake the activities relating to the notification and may continue to undertake such activities subject to any order made by the Board.
b. The acknowledgement issued shall in no way absolve the person from complying with other written laws governing such living modified organism and its importation.
V. NOTIFICATION PROCESS
Contained use activities which are exempted from the notification
Biosafety (Approval and Notification) Regulations 2010 (page 20-27).
VI. APPLICATION FOR NOTIFICATION PROCEDURES
Please use the IBC checklist form as the guide in completing required fields to be submitted to IBC.
b. Example Form E: Click Here
c. Standard Operating Procedures: Click Here
Please sent your application to This email address is being protected from spambots. You need JavaScript enabled to view it.
EXEMPTION
The First Schedule of the Biosafety (Approval and Notification) Regulations 2010 allows exemptions for some types of techniques and contained use activities in relation to LMO posing a very low risk (i.e.contained research activities involving very well understood organisms and processes for creating and studying LMO):
a. Exempted activities should be carried out under conditions of standard laboratory practice.
b. Appropriate biosafety levels as according to Second Schedule of the Biosafety (Approval and Notification) Regulations 2010 should be used for the exempted activities and personnel should have appropriate training.
c. Principal Investigators who believe that the work falls into any of the exemptions should nevertheless notify their IBC of the proposed project.
d. The IBC may review all submitted research projects to determine their exemption or non-exemption status.
• Exemption List (First Schedule) : Click Here
• Exemption List (by Minister) : Click Here
OTHER FORMS
a. IBC Assessment of Project Proposal Involving Modern Biotechnology Activities (ANNEX 2)
b. Notification for export of LMO (Form F): Click Here
c. Incident reporting form (ANNEX 3) : Click Here
d. Occupational disease / exposure investigation (ANNEX 4): Click Here
e. Project extension & notice of termination (ANNEX 5) : Click Here
Please sent your application to This email address is being protected from spambots. You need JavaScript enabled to view it.
RESOURCES
1. Website Department of Biosafety (URL- http://www.biosafety.gov.my/)
2. Biosafety Clearing House (URL- http://bch.cbd.int/)
3. Cartagena Protocol (URL-http://bch.cbd.int/protocol/background/)
LAW AND REGULATION
1. Biosafety Act 2007 (Act 678), Akta Biokeselamatan 2007 (Akta 678)
i Biosafety (Approval and Notification) Regulations 2010
ii The Biosafety (Sampling Procedures) Regulations 2018
iii The Biosafety (Compounding of Offences) Regulations 2018
2. Guidelines for Contained Use Activity of Living Modified Organism (LMO)
3. User's Guide to The Biosafety Act and Regulations
4. Guidelines For Institutional Biosafety Committees (IBC)
5. Biosafety Guidelines: Risk Assessment of Genetically Modified Microorganisms